INTRODUCTION
MATERIALS AND METHODS
Plant materials and growth conditions
Imagery data collection in root
Statistical Analysis
RESULTS AND DISCUSSION
Variability of root morphological traits
CONCLUSION
SUPPLEMENT
Introduction
Adzuki bean (Vigna angularis) contains enriched carbohydrates and proteins, so it regards as an important annual legume crop that is widely cultivated in China, Japan, Korea and India (Hori et al., 2006). It is widely used as a functional food due to its various health benefits (Han et al., 2015). It has been studied for its numerous health benefits such as detoxification, antioxidant and anti-obesity properties (Amarowicz et al., 2008; Kitano‐Okada et al., 2012).
Due to the consequences of climate change, there have been alterations in the rainfall patterns in Korea, which has increased the occurrence of drought from spring to summer (Park et al., 2012). Such climatic condition has affected the planting date of upland crops such as soybean, sesame and adzuki bean (Chun et al., 2016). Cultivated adzuki bean is subjected to severe drought periods, and it has been reported that they are sensitive to soil moisture content during early vegetative growth stages (Chun et al., 2021). Adzuki bean is one of the important legume crops in Korea, so it is necessary to understand its morphology and adaptation to water stress (Chun et al., 2021). It has been reported that drought stress in adzuki beans can inhibit root and shoot growth and physiological and biochemical characteristics such as the potential activity of photosystem II, photochemical efficiency in different adzuki bean cultivars in the seedling stage (Luo et al., 2014).
In general, root morphological trait (RMT) and root architectural trait (RAT) involves in the productivity of most crops, because these traits are not only important in water uptake but also participate in inorganic nutrient uptake from soil (Manschadi and Christopher, 2006). Therefore, understanding RMT and RAT in various crops are necessarily required for improving water and nutrient uptake as well as enhancing the efficiency of water and nutrients (Prince et al., 2017; Kim et al., 2020; Tripathi et al., 2021b). Among the various traits associated with mitigation of drought stress in crops, RMT and RAT are considered to be the most vital traits that enables plants to uptake water from deeper soil layers (Vadez et al., 2008). Despite the fact that RMT and RAT, both are very important traits for mitigating drought stress through water and nutrient uptake, however, few studies on RMT and RAT analysis have been conducted in cultivated soybean (Glycine max L.) and wild soybean (Glycine soja Siebold & Zucc.) (Kim et al., 2021a; Kim et al., 2021b; Tripathi et al., 2021a). On the other hand, there was no related research on adzuki beans for detailed RMT and RAT evaluation reported. Only root morphology and spatial distribution depending on different moisture contents in soil were conducted in an adzuki bean cultivar, which was named “Arari” (Chun et al., 2021). It is well known that perhaps the root is the very first plant part to respond to stress under various soil environmental conditions such as drought, salinity and flooding stress (Kunert et al., 2016). As a result , recently, the improvement of RMT and RAT has been identified as target traits in he breeding program to enhance the resistance to unfavorable soil and weather conditions (Kim et al., 2020). For all these reasons, current breeding efforts in leguminous crops are centered on identifying superior root traits genotypes and then using those genotypes as breeding material for crop improvement (Vadez et al., 2008; Wang et al., 2019). Previously our research group assessed RMT and RAT in cultivated soybean (Kim et al., 2021a; Tripathi et al., 2021a), and wild soybean (Kim et al., 2021b) using imagery data to supply soybean breeding programs for the improvement of root morphological traits. Our research group used this information for developing breeding populations and the identification of QTLs responsible for root traits in cultivated soybean. Despite the fact that adzuki beans are a significant upland crop in South Korea, research into RMT and RAT evaluation in adzuki beans has received little attention. Thus, in this experiment, we evaluated the RMT and RAT among the 22 adzuki bean accessions using well established root imaging analysis technology to uncover new new information about RMT and RAT in adzuki beans.
MATERIALS AND METHODS
Plant materials and growth conditions
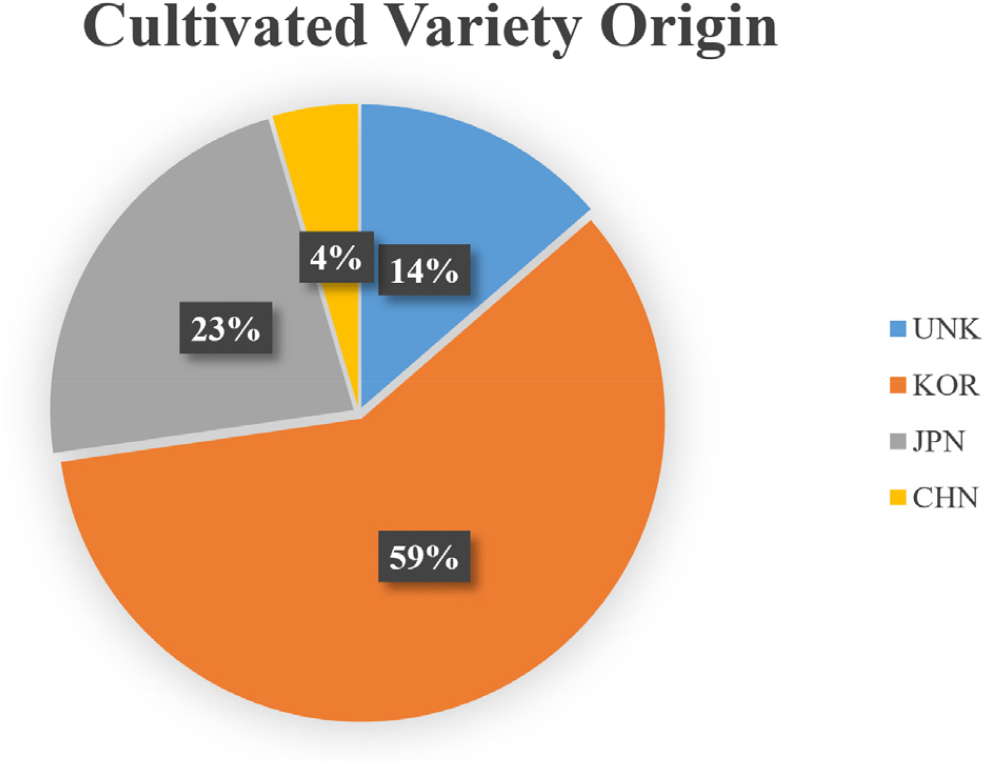
Adzuki bean 22 cultivars which were donated from NAC to the Rural Development Administration in South Korea were used in the experiment (Supplementary Table 1). Among 22 accessions, 13 accessions were collected in South Korea (KOR) and rest were collected in the Asia region [3 accessions from Afghanistan (UNK), 5 accessions from Japan (JPN), 1 accession from China (CHN)] (Fig. 1). All seeds were subjected to a treatment process (seed scarification) to increase the germination rate by slightly scraping the ends of the seeds using a nail clipper before planting. The experiment was conducted in a greenhouse which was in Kyungpook National University Research Center, Daegu. Two scarified seeds were sown in polyvinyl chloride (PVC) pipes [6 cm (diameter) × 40 cm (height)] containing horticultural soil used for seed germination and plant proper growth (Tobirang, Baekkwang Fertility, South Korea). To use PVC pipe as a pot, one side of the end was covered with non-woven fabric, then the soil was poured into the other side of the PVC pot. The experiment was carried out approximately for a month (from July 25th, 2021 to August 28th, 2021) and the average temperature and humidity of a day were around 32°C ± 3°C and 67% ± 5%, respectively. All PVC pots were randomly placed at a greenhouse and an experiment was conducted with 5 replications (n =1).
Table 1.
Analysis of variance of six root morphological traits.
| Parameters | Source | DF | Type III SS | Mean Square | F Value | Pr > F |
| TRL | cultivar | 21 | 1803240 | 85868.59 | 3.62 | <.0001 |
| rep | 4 | 143305.8 | 35826.45 | 1.51 | 0.2089 | |
| SA | cultivar | 21 | 17931.03 | 853.8584 | 3.84 | <.0001 |
| rep | 4 | 1754.267 | 438.5667 | 1.97 | 0.1088 | |
| AD | cultivar | 21 | 0.095002 | 0.004524 | 5.11 | <.0001 |
| rep | 4 | 0.005913 | 0.001478 | 1.67 | 0.167 | |
| NT | cultivar | 21 | 13484540 | 642120.9 | 2.69 | 0.0011 |
| rep | 4 | 777859.2 | 194464.8 | 0.82 | 0.5194 | |
| LAL | cultivar | 21 | 0.013014 | 0.00062 | 5.34 | <.0001 |
| rep | 4 | 0.001569 | 0.000392 | 3.38 | 0.0139 | |
| LAD | cultivar | 21 | 0.241331 | 0.011492 | 6.32 | <.0001 |
| rep | 4 | 0.023153 | 0.005788 | 3.19 | 0.0185 |
Imagery data collection in root
When the plants reached the 2nd or 3rd trifoliate leaves stage, the roots were harvested. To collect root samples, The sieve is prepared, then PVC pots pour into a sieve. We carefully took out plant samples from dumped soil, then removed the shooting part with scissors. After that, root samples were washed with clean tap water to remove soil particles from the root. Washed-root samples were immediately kept in a zip-lock bag containing 10 ml of clean tap water to prevent root drying. During samples collection, a zip-lock bag was kept in an icebox, then all root samples were kept in a refrigerator until root analysis. Root image was captured at scanner (Expression 12000XL, Epson, Japan) coupled with transparent tray (length: 30 cm; width: 20 cm). We carefully poured clean root samples with tap water on the transparent tray, then again removed tiny soil particles or root debris using tweezers to collect a clean image. The acquired image was analyzed via root image analysis software (WinRHIZO Pro Software, Regent Instruments Inc., Canada) (Fig. 2). WinRHIZO Pro classifies roots into various diameter classes. In WinRHIZO software, various RMT and RAT are possible to analyze. TRL is the total length of the whole root system that is included in all the diameter classes. Similarly, AD is the average of diameter classes of all root segments. WinRHIZO also has a link analysis function, which is used for the study of morphology and basic connectivity of roots segments. A link is a root segment between a fork and a tip or two tips. For the entire image, this function processes the total number of links, LAL, LAD and other parameters. Among them, TRL, SA, AD and NT was selected to figure out distinguish of RMT among adzuki bean, while link LAL, and LAD was selected to understand the difference of RAT in adzuki beans. Detailed definition of each root trait is described in Table 2.
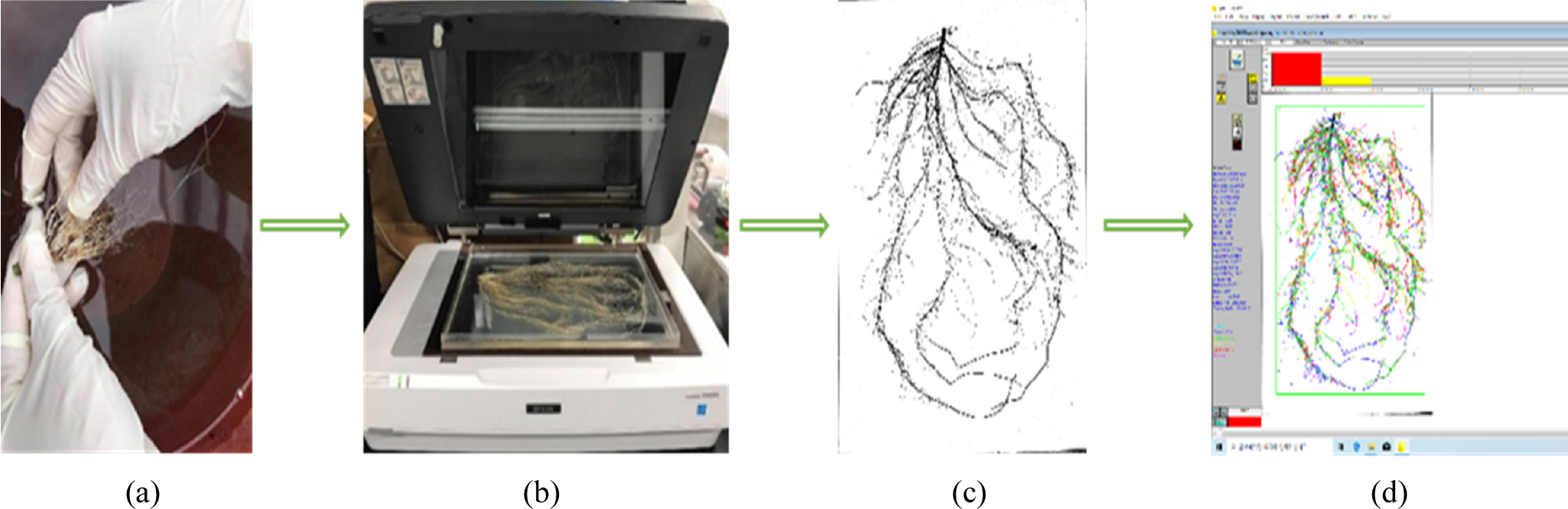
Fig. 2.
Scheme of root samples analysis. In this figure, (a) indicated root washing with tap water; (b) showed the process of root sample scanning where clean root samples were placed onto a transparent acrylic tray containing clean tap water; (c) is an image after the scan; and (d) presents the analyzed root morphological and architectural traits by WinRHIZO pro software.
Table 2.
Description of the root traits analyzed in the study using WinRHIZO software.
Statistical Analysis
To determine statistical significance, we conducted an ANOVA test and the correlation analysis was performed with R studio version 1.3.1093 to investigate the relationship among various root phenotypes. Frequency distribution histograms for all root traits among the 22 adzuki bean cultivars and descriptive statistics or inferential statistical analysis were computed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Figures were generated using Microsoft Excel (2013) or R studio version 1.3.1093.
RESULTS AND DISCUSSION
Variability of root morphological traits
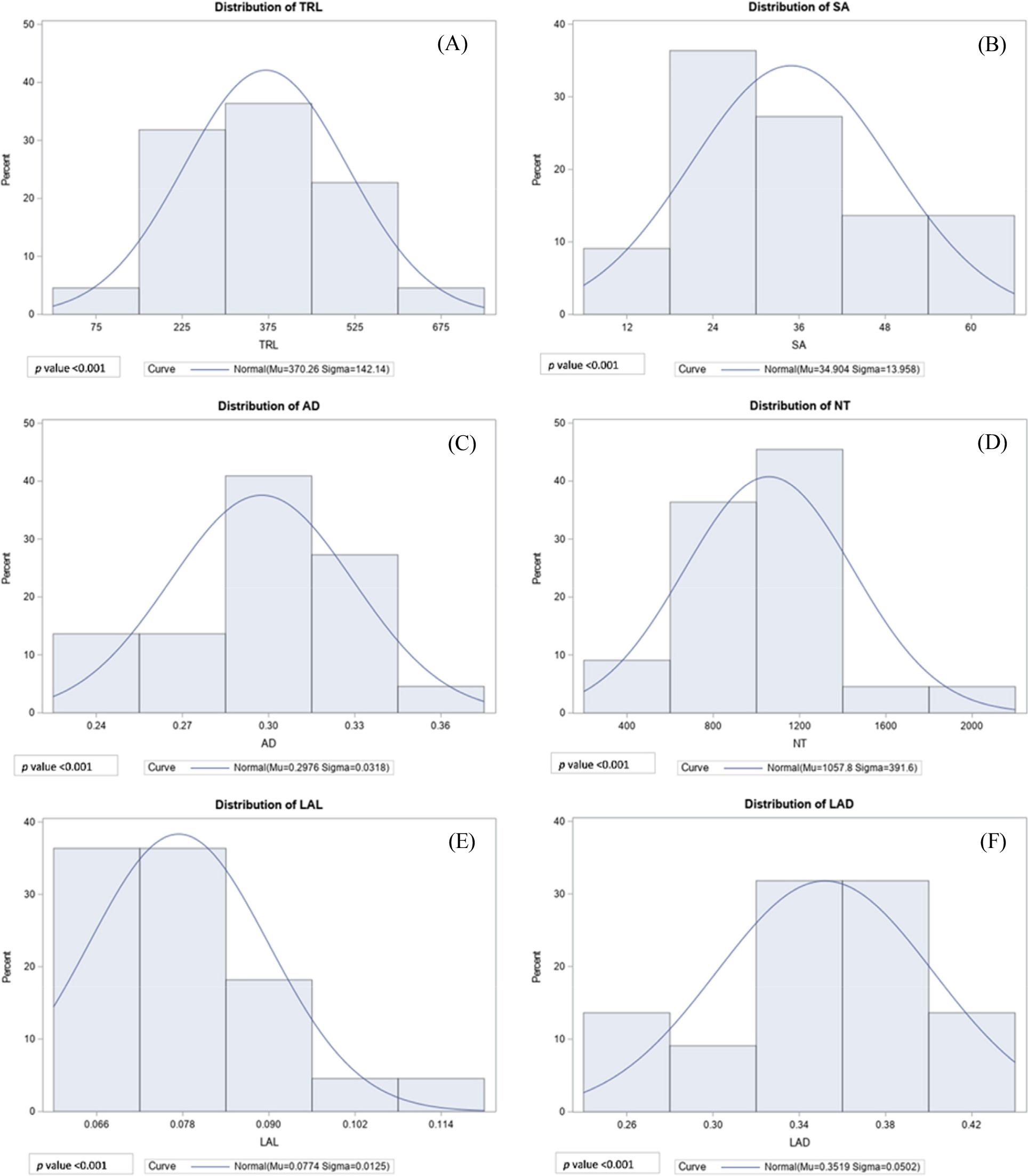
The root is an important part of the plant in terms of response to drought stress, it can initiate a response mechanism at a physiological or genetic level (Kim et al., 2020). We reported key root morphological traits of 22 adzuki bean cultivars, such as TRL, SA, AD, NT, LAL and LAD. The combined Analysis of Variance (ANOVA) demonstrated high significant differences among the cultivars for all the root traits under study (viz., TRL, SA, AD, NT, LAL and LAD) as shown in Table 1. The data of all root morphological traits were normally distributed when checked visually across all the cultivars (Fig. 3). The statistical variation as descriptive statistics in root traits data is depicted in Supplementary Table 2. The RMT, TRL, SA, and AD were observed with a range of 91.46-717.14 cm, 7.74-60.68 cm2, and range 0.24-0.36 cm respectively (Supplementary Table 2). The values range (Supplementary Table 2) suggests that there is a significant difference between the highest and lowest cultivars. In addition, among these three traits, SA showed the highest variation with a coefficient of variation (CV) near about 40% followed by SA and AD 38.39% and 10.70% respectively. Similarly, RAT, such as NT, LAL, and LAD observed with a range of 260.50-2036.75 number, 0.06-0.12 cm and 0.25-0.44 cm respectively. NT showed the highest CV with 37.02%, followed by LAL and LAD with 16.12 and 14.26% respectively. As the skewness is in between -1.96 and +1.96, hence the distribution of the data is observed to be univariate and as kurtosis values are less than 3, except for LAL, the dataset is observed to follow a normal distribution and histograms of all root traits excluding for LAL indicate their normal distribution (Supplementary Table 2, Fig. 3).
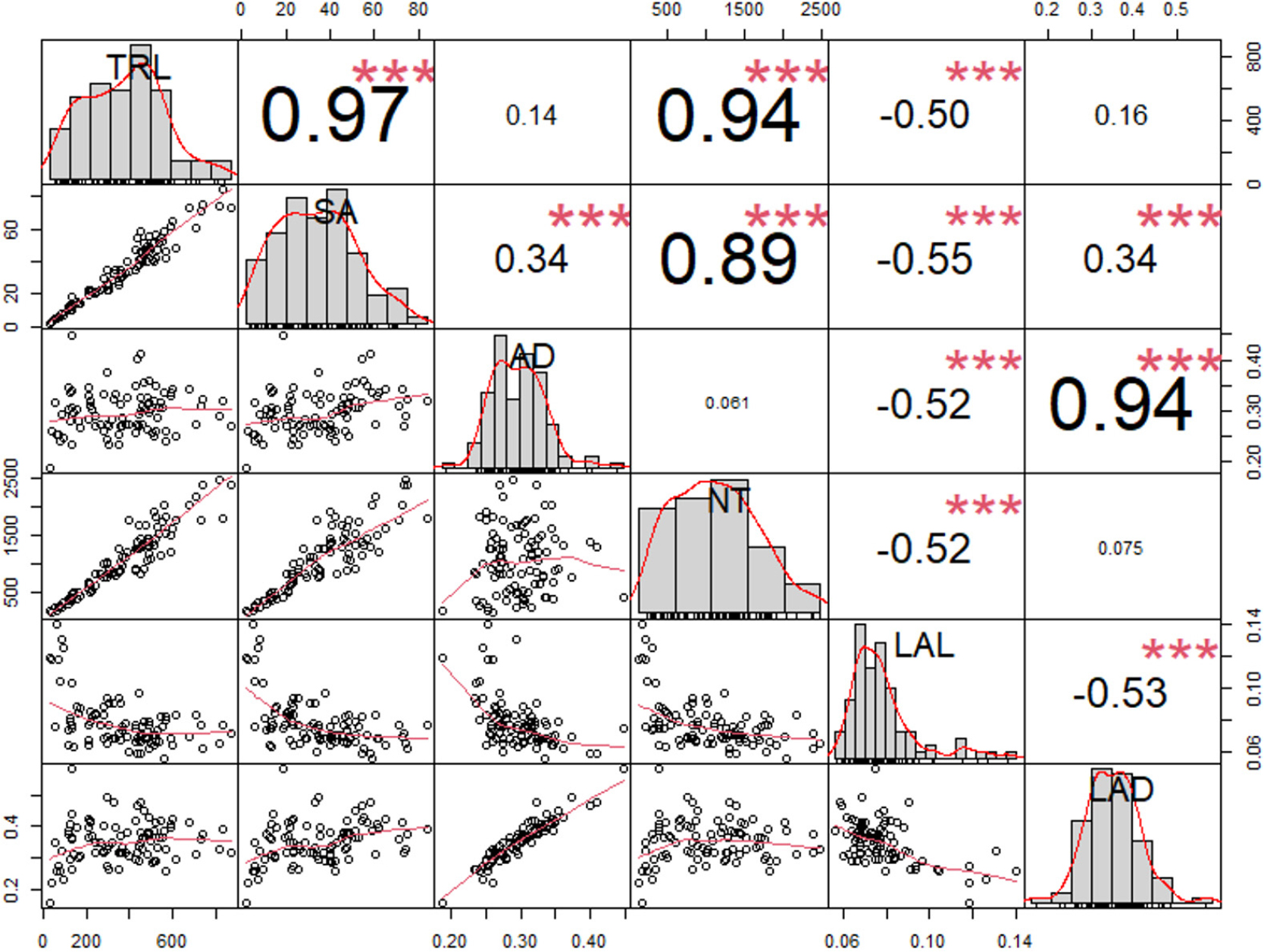
In plants, RMT and RAT are both known to be significant factors for root growth, symbiotic relationships with microflora, and resistance to stresses such as osmotic and nutrient stress. As we mentioned above, investigated six root traits in this experiment were included in RMT and RAT as root phenotyps. The correlation between RMT and RAT was investigated in major crops such as soybean (Tripathi et al., 2021a) and maize (Long et al., 2020), but not in Adzuki bean. As a result, we conducted a correlation test among root traits. We used a procedure called PROC FREQ to calculate probabilities of the normality, and F distributions by SAS software. In this study, based on the correlation analysis, we found that root length- related traits like TRL and SA had the highest correlation values when compared to other trait. TRL had a very strong positive phenotypic correlation at 0.05% significance level (r=0.97) with SA (Fig. 4). Similarly, we found strong positive correlation with TRL (r=0.94) and SA (r=0.89) (Fig. 4) at 0.05% significance level. On the contrary, LAL showed significant negative correlations with NT (r=-0.52), AD (r=-0.51), SA (r=-0.55) and TRL (r=-0.50) (Fig. 3) respectively.
In other legume crops like soybean, root phenotyping of numerous cultivars have been reported (Kim et al., 2021a). Furthermore, during the germination stage, adzuki bean germplasm was evaluated and screened for drought tolerance (Zhu et al., 2019). The novelty of this study is that we used 22 popular and widely grown adzuki bean cultivars to evaluate root morphological and architectural traits in the vegetative stage. As root morphological and architecture traits in plants are considered to be very important traits for crop improvement which is often influenced by environmental conditions as well as some linked traits. To withstand drought stress, a plant must have a strong root system with longer root lengths that can absorb water from deeper soil levels during drought (Turner et al., 2001; Kumar et al., 2012). Thus, we aim to use the existing set of extremely promising cultivars to generate even higher and better root traits genotypes or crop ideotypes like improved RMT and RAT adzuki beans, which probably withstand extreme environmental conditions greater than popular adzuki bean check cultivars and ultimately contribute to high crop production. Both root elongation and differentiation have been considered important processes in the aspect of how plants respond to external environmental factors that hinder plant growth (Zheng et al., 2016). Numerous highly conserved miRNA profiles were studied from the primary root tips in soybean, which were found to be differentially regulated in response to drought stress (Zheng et al., 2016). This reflects the importance of a number of tips in the roots in response to drought stress, indicating that NT could be a useful trait to indicate the potential for water and nutrient uptake during the response to drought stress. As per our findings, TRL has a very strong positive correlation with NT. As a result, cultivars with high TRL, SA, NT, LAL, and LAD may be considered for breeding traits, particularly to develop drought-tolerant cultivars. Genetic variation in legumes has been reported to affect the number of metaxylems in roots which can alter the water movement throughout the root system and into the shoots, therefore the genes that regulate the root system architecture can be prioritized for cultivar improvement and stress tolerance in adzuki bean (Prince et al., 2017; Xiong et al., 2021).
From the total of 22 adzuki bean cultivars, we listed the top and bottom 5 cultivars for every root morphological trait under consideration in this study (Table 3). According to the results, ‘IT 236657’ demonstrated the highest TRL, SA and NT (Table 3). Likewise, ‘IT 236169’ showed the lowest value for TRL, SA and NT (Table 3). Similarly, ‘IT 236171’ showed the lowest AD and LAD (Fig. 4). The root morphological traits evaluation of azuki beans have not yet been fully reported. Therefore, our results would be useful in a breeding program for developing root reinforced-adzuki bean.
Table 3.
List of top and bottom five cultivars for each root trait.
| TRL (cm) | SA (cm2) | AD (mm) | NT (number) | LAL (cm) | LAD (mm) | |
| Top 5 cultivars for each root trait |
717.13 IT 236657 |
60.68 IT 236657 |
0.36 IT 236659 |
2036.75 IT 236657 |
0.12 IT 236169 |
0.43 IT 209444 |
|
530.47 IT 236658 |
58.48 IT 236659 |
0.34 IT 209444 |
1481.50 IT 236658 |
0.009 IT 236171 |
0.43 IT 236659 | |
|
526.54 IT 236659 |
56.10 IT 236658 |
0.336 IT 236658 |
1367.80 IT 236659 |
0.085 IT 270036 |
0.42 IT 154837 | |
|
506.65 IT 229428 |
48.40 IT 229428 |
0.334 IT 154837 |
1365.75 IT 238547 |
0.086 IT 294673 |
0.39 IT 236658 | |
|
469.05 IT 216355 |
46.70 IT 216355 |
0.325 IT 221513 |
1341.75 IT 229071 |
0.084 IT 229428 |
0.37 IT 216355 | |
| Bottoms 5 cultivars for each root trait |
91.46 IT 236169 |
7.74 IT 236169 |
0.23 IT 236171 |
260.50 IT 236169 |
0.064 IT 215376 |
0.24 IT 236171 |
|
203.79 IT 294673 |
17.82 IT 236171 |
0.24 IT 240375 |
584.00 IT 294673 |
0.065 IT 236658 |
0.277 IT 240375 | |
|
224.70 IT 154837 |
19.31 IT 294673 |
0.25 IT 270036 |
602.00 IT 154837 |
0.066 IT 236659 |
0.278 IT 236169 | |
|
226.57 IT 236171 |
22.38 IT 182073 |
0.267 IT 236657 |
642.40 IT 209444 |
0.067 IT 221513 |
0.29 IT 270036 | |
|
253.64 IT 182073 |
24.07 IT 154837 |
0.268 IT 236169 |
691.00 IT 236171 |
0.068 IT 238547 |
0.31 IT 236657 |
CONCLUSION
We found a significant difference between the cultivars for most of the measured root morphological traits. The highest variation was observed for the SA, followed by the TRL and NT among the tested cultivars. Furthermore, a strong positive correlation was found between TRL and SA. Whereas, LAL showed significant negative correlations with NT, AD, SA and TRL. This research study would be extremely useful in identifying target root traits for the adzuki bean breeding approach in the future for developing climate-resilient adzuki bean, particularly for drought stress. In addition, it would be desirable to find in further study how these root traits affect the adzuki bean yield and other biotic and abiotic stress tolerance under greenhouse as well as field conditions.
SUPPLEMENT
Supplementary Table 1.
List of adzuki bean cultivars.
Supplementary Table 2.
Descriptive statistics table for six root traits in 22 cultivars.
| Traits | Range | Mean | SDa | CV (%)b | Skewness | Kurtosis | Pr value |
| TRL | 91.46–717.14 | 370.26 | 142.14 | 38.39 | 0.33 | 0.33 | 0.001 |
| SA | 7.74–60.68 | 34.90 | 13.96 | 39.99 | 0.19 | -0.52 | 0.001 |
| AD | 0.24–0.36 | 0.30 | 0.03 | 10.70 | -0.01 | -0.50 | 0.001 |
| NT | 260.50–2036.75 | 1,057.76 | 391.60 | 37.02 | 0.23 | 0.70 | 0.001 |
| LAL | 0.06–0.12 | 0.08 | 0.01 | 16.12 | 1.92 | 5.12 | 0.001 |
| LAD | 0.25–0.44 | 0.35 | 0.05 | 14.26 | -0.27 | -0.24 | 0.001 |